The manufacture of biopharmaceutical agents such as therapeutic monoclonal antibodies, vaccines and cell-based therapies like CAR-T cells require the highest quality control against the contamination of adventitious agents. These pathogens can enter the manufacturing process at multiple stages and impact yield and safety. Current methods for detection of adventitious agents are expensive, lack sensitivity and have long turn around times to results. Moreover, many of the current adventitious agent testing methods rely heavily on animal-based testing to monitor for infection response.
NGS methods are increasingly being explored for as a faster, more accurate and cost-effective approach than traditional methods to identify biocontaminants. However due to the highly complex workflows of NGS and the potentially low levels of contamination, there is a strong need for a sample-level process control that can ensure the assay is meeting its sensitivity performance specifications across all targets. Since the desired result is the absence of contaminating agents, how can the absence of detection be assured to be the result of a true negative vs. performance drift?
SNAQ-SEQ NGS spike-in standards are an ideal tool to accurately measure the performance of each sample in the test. Large multiplexed mixtures of DNA and RNA-based standards to the targeted adventitious agents assay can be quantitatively added to the sample lysates prior to extraction, and serve as a quantitative measure of performance for EVERY sample by following the entire workflow through to sequence detection. Detection sensitivity using SNAQ-SEQ can be assured to as low as 10 copies of virus in many cases.
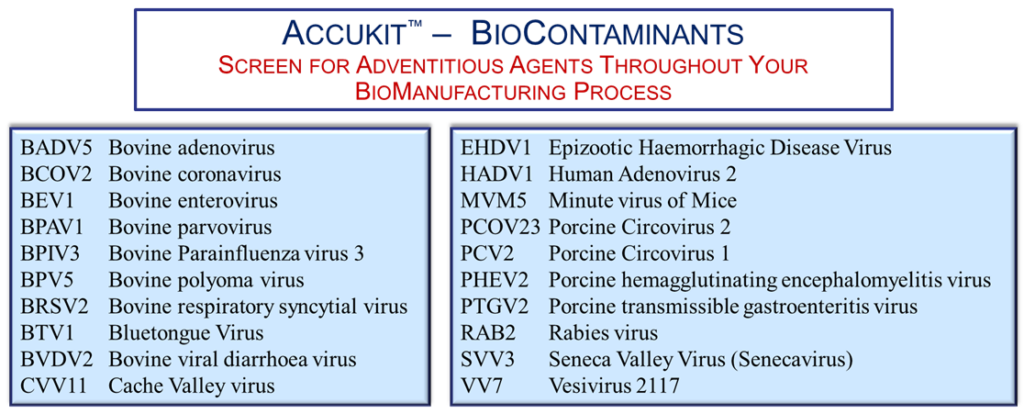
Additional Resources:

