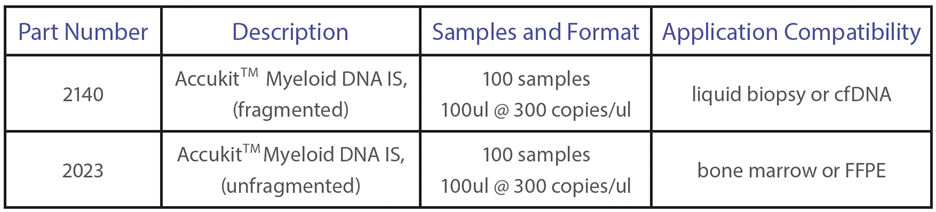
AccukitTM Myeloid DNA IS
SNAQTM-SEQ Internal Standards for MRD

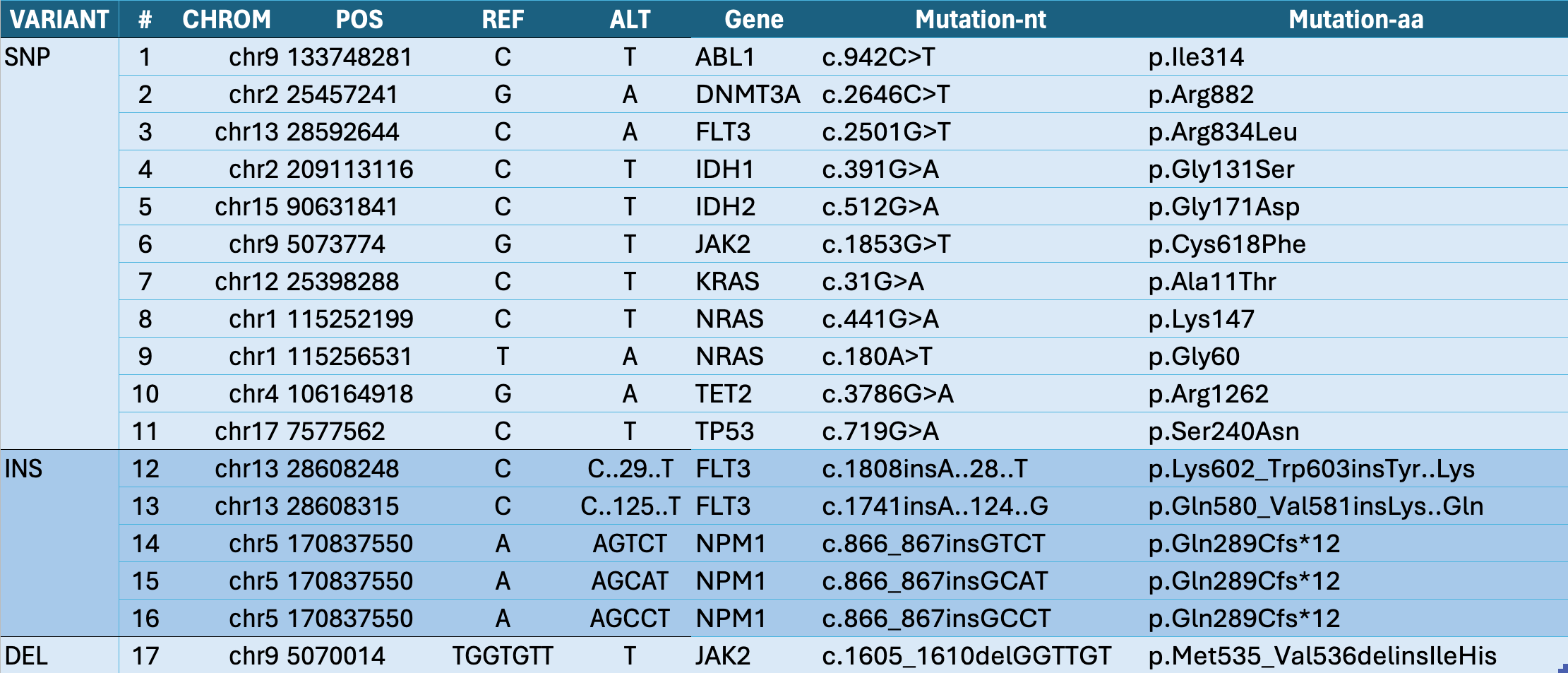
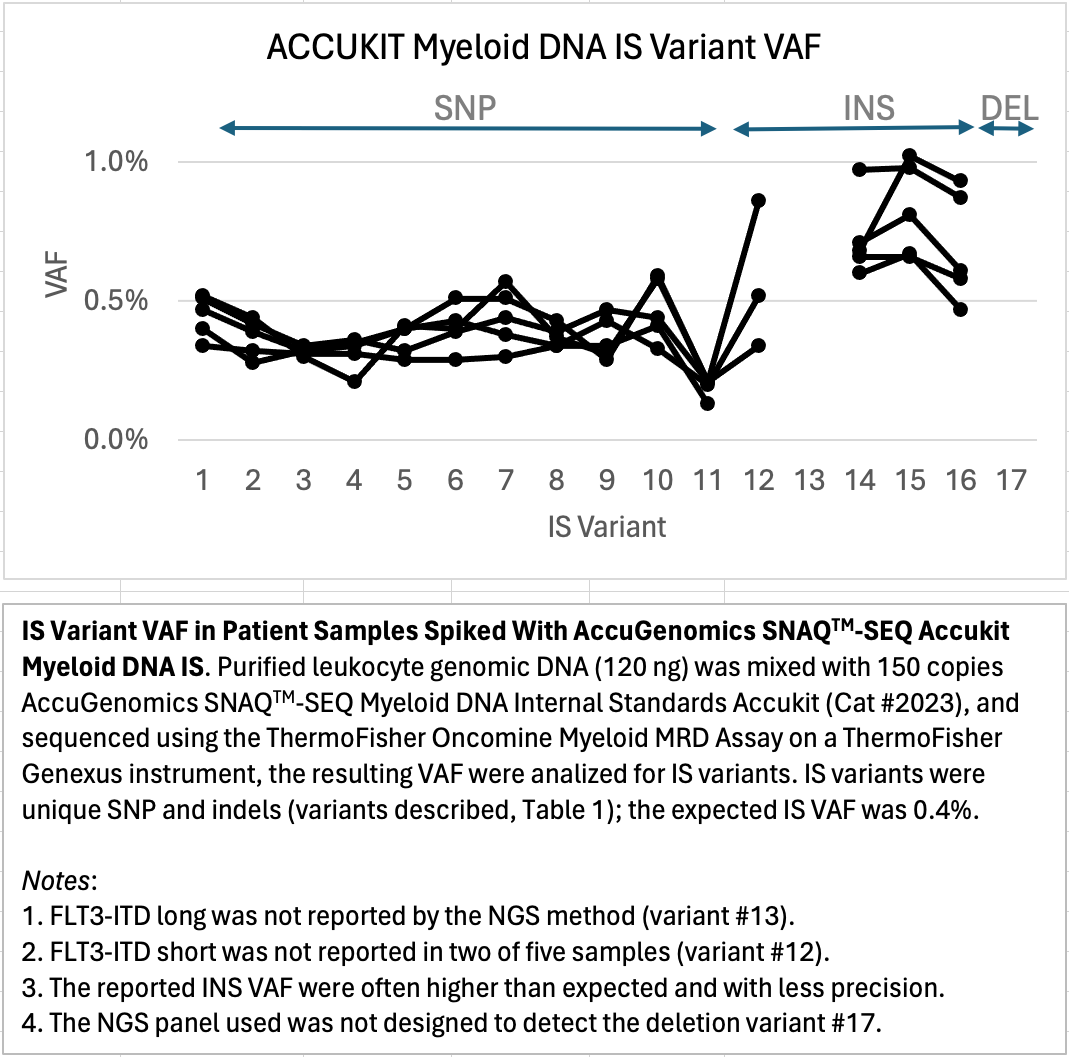
Actionable AML Internal Standards validate the assay and sample performance for every variant, to confirm the true Limit of Detection for each sample tested.
Download the Accukit™ Myeloid DNA IS Product Sheet

Standardized Nucleic Acid Quantification for Sequencing (SNAQTM-SEQ) is a critical QC method to provide variant specific sensitivity controls for therapeutically relevant Acute Myeloid Leukemia (AML) targets in each sample. Mixtures of synthetic DNA created to mimic the sample regions of interest are added to each sample prior to NGS library preparation; they covary through the complex chemistry, flow cell detection and bioinformatics workflow, mirroring the samples native target template to provide the ideal assay run control. Internal standards (IS) bring the reference material directly into the sample, eliminating the need for external run controls for more cost-efficient reagent and flow cell utilization, while also providing direct QC of the sample.
AccukitTM Myeloid DNA IS are added to each sample as a single reagent addition, represent genomic regions with clinically relevant cancer driver variants, and are compatible with hybrid capture and amplicon-based library prep chemistries. Internal standards enable true limit of detection (LOD) assurance, which ensures that a given abundance input will be detected (if present). This means that the assay is highly sensitive and can detect low levels of target sequences with high accuracy, even in samples with low tumor burden. AccukitsTM are available pre-fragmented for use with blood and plasma (cfDNA), or unfragmented for FFPE or tissues; they are easily compatible with your existing workflow, platform, and analysis pipeline.
AccukitTM Myeloid DNA IS improves the accuracy of measurable residual disease (MRD) detection for Acute Myeloid Leukemia (AML), and provides a high-quality and universal solution to improve the value of monitoring targeted next-generation sequencing (NGS) for AML patients. Quantitative NGS assays provide important insights into disease progression for the best patient outcomes, and the use of SNAQTM-SEQ Internal Standards (IS) enable a comprehensive and standardized approach to AML testing by sequencing.
For more information: [email protected]
FOR RESEARCH USE ONLY
